Lewis dot of Nitrogen Monoxide (NO)
A simple procedure for writing Lewis Structures is given in a previous article entitled Lewis Structures and the Octet Rule. Several worked examples relevant to this procedure were given in previous posts please see the index page Lewis Structures & the Octet Rule - Theory & Examples.
Another example for writing Lewis structures following the above procedure is given in this post. The Lewis structures of Nitrogen Monoxide NO are drawn.
Let us consider the case of NO. NO is a free radical and is an important intermediate in the chemical industry. Nitric oxide is a by-product of combustion of substances as in automobile engines, fossil fuel power plants, and is produced naturally during the electrical discharges of lightning in thunderstorms. In mammals including humans, NO is an important cellular signaling molecule involved in many physiological and pathological processes. The vasodilating antihypertensive drug minoxidil contains an NO moiety and may act as an NO agonist. Likewise, Sildenafil citrate, popularly known by the trade name Viagra, stimulates erections primarily by enhancing signaling through the nitric oxide pathway in the penis.
Let us draw the Lewis dot structures of Nitrogen Monoxide (NO).
Step 1: Connect the N with the O atom with single bonds
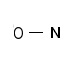
Step 2: Calculate the # of electrons in π bonds (pi bonds, multiple bonds) using formula (1) in the article entitled “Lewis Structures and the Octet Rule”.
Where n in this case is 2 since NO consists of two atoms.
Where V = 5 +6 = 11 , V is the number of valence electrons of the ion.
Therefore, P = 6n + 2 – V = 6 * 2 + 2 – 11 = 3 So, there is 1 double bond and an electron.
Step 3 & 4: The Lewis dot resonance structures of Nitrogen Monoxide (NO) are as follows:

- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)