A simple procedure for writing Lewis dot structures was given in a previous post entitled “Lewis
Structures and the Octet Rule”.
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Let us consider
the case of acetamide CH3CONH2
Acetamide has many uses and applications such as:
- As a general solvent (molten acetamide is excellent solvent for many organic and inorganic compounds)
- in biocides
- in preparation of cosmetics and hypnotics,
- in various organic and inorganic syntheses,
- as a drug intermediate in the manufacture of ampicilline, cephaclor, cephalexin, cephradine, enalapril
- as an antacid in lacquers and cosmetics
- as a plasticiser in leather and coatings
Step 1: Connect the atoms with
single bonds.
 |
|
Fig. 1 : Connect the atoms
of acetamide
with single bonds.
|
Step 2: Calculate the # of electrons in π bonds (pi bonds, multiple bonds) using formula (1):
Where n in this
case is 4 since CH3CONH2
consists of nine atoms but five of them is H.
Where V = (1*3 +
4 + 4 + 6 + 5 + 1*2) = 24
Therefore, P = 6n
+ 2 – V = 6 * 4 + 2 – 24 = 2 So there are 2 π electrons (pi electrons)
in CH3CONH2 and therefore1 double bond must be added to the structure of Step 1.
Step 3 & 4: One
double bond must therefore be placed in the structure shown in Fig 1:
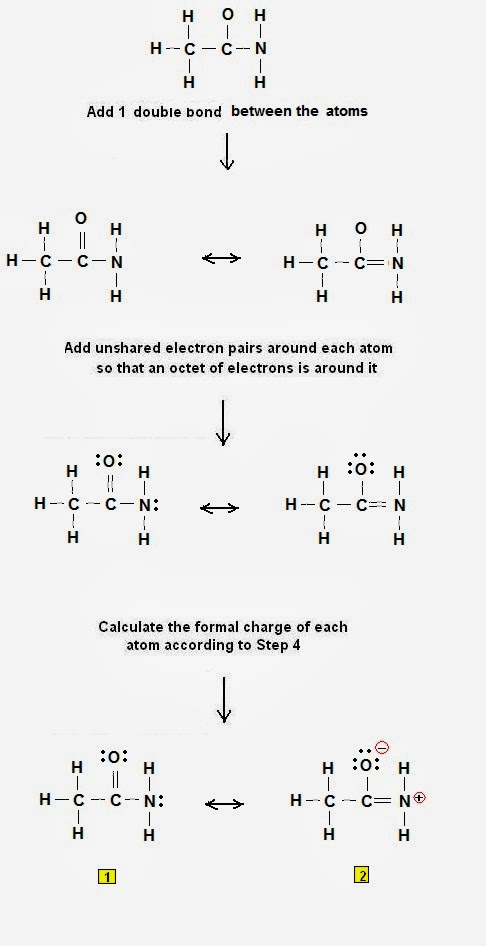 |
|
Figure 2: Lewis structures
of acetamide
|
Prof. Prem raj Pushpakaran writes -- 2025 marks the birth centenary year of John Pople, and let us celebrate the occasion!!! https://worldarchitecture.org/profiles/gfhvm/prof-prem-raj-pushpakaran-profile-page.html
ReplyDelete