Lewis Electron Dot Structure of sulfur trioxide SO3
A simple procedure for writing Lewis Structures was given in a previous article entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Consider the case of sulfur trioxide, SO3. SO3 is used for the industrial production of sulfonates - organic compounds with a sulfur carbon bonds - when it reacts with organic compounds. A well known and mass produced sulfonate is sodium dodecylbenzenesulfonate (also commonly referred to as linear alkylbenzene sulfonate or LAS) which is a series of organic compounds with the formula C12H25C6H4SO3Na. It is a colourless salt with useful properties as a surfactant. It is usually produced as a mixture of related sulfonates. LAS is a major component of laundry detergent.
SO3 plays also a major role in acid rain formation since it is hydrated in the atmosphere to form sulfuric acid and sulfates at an increased reaction rate due to the rather high water content in the troposphere.
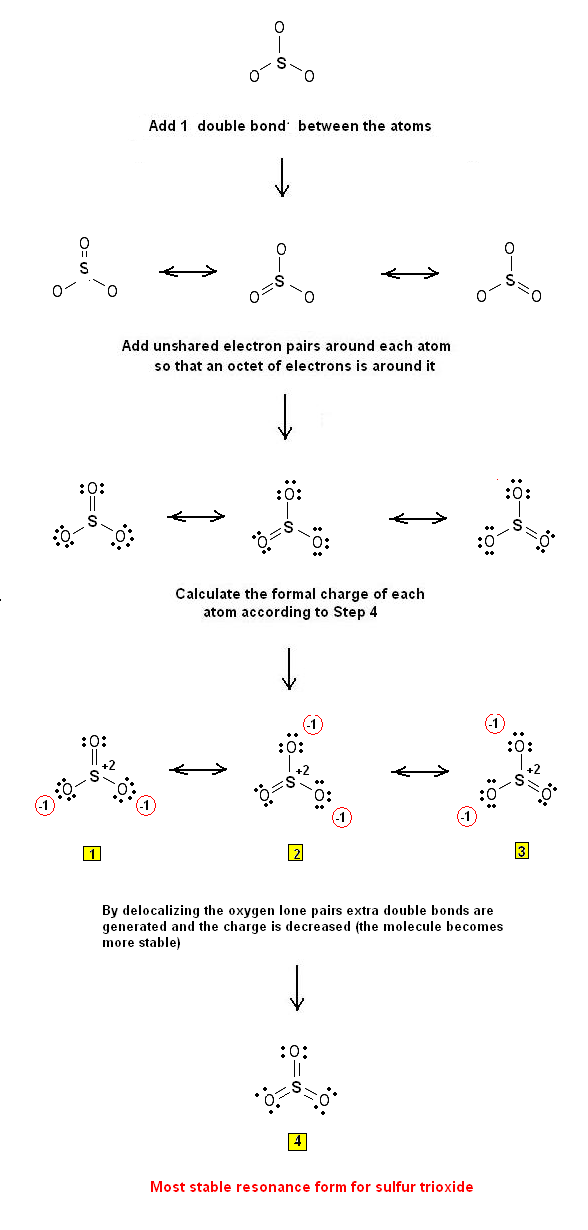
The following steps are used to draw the Lewis Structure(s) of SO3:
Step 1: The central atom will be the S atom since it is the less electronegative. Connect the atoms with single bonds:

Step 2: Calculate the # of electrons in p bonds (multiple bonds) using formula (1) :
Where n in this case is 4 since SO3 consists of four atoms.
Where V = (6 + 6 + 6 + 6) = 24 Therefore, P = 6n + 2 – V = 6 * 4 + 2 – 24 = 2 So there are 2 p electrons in SO3 and therefore 1 double bond must be added to the structure of Step 1.
Step 3 & 4: One double bond must therefore be placed through the 3 S-O bonds. Therefore, the Lewis structures of SO3 are as follows:
The above simple procedure for drawing Lewis electron dot structures is also shown in the following video:
Relevant Posts
Lewis Structures|Octet Rule: A Simple Method to write Lewis Structures
Lewis structure of SO2 – Simple Procedure for drawing Dot structures
Lewis Dot Structure of the sulfite ion SO3-2
References
- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)
Key Terms
resonance structures of sulfur trioxide SO3, Lewis electron structures of SO3, chemical formula of SO3, simple method for drawing Lewis structures of SO3,
No comments:
Post a Comment