Canonical Lewis Structures of OCN-
A simple procedure for writing canonical Lewis Structures was given in a previous post entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Let us consider the case of the cyanate ion OCN- . The cyanate ion is an ambidentate and a bridging ligand. The Lewis structures of the cyanate ion are drawn in 4 steps using the above method.
Step 1: Connect the atoms with single bonds.
Step 2: Calculate the # of electrons in pi bonds (multiple bonds) using formula (1):
Where n in this case is 3. Where V = (6 + 4 + 5) – (-1) = 16 , V is the number of valence electrons of the molecule.
Therefore, P = 6n + 2 – V = 6 * 3 + 2 – 16 = 4 So, there are : 2 double bonds or a triple bond.
Step 3 & 4: The resonance structures of OCN- are as follows:
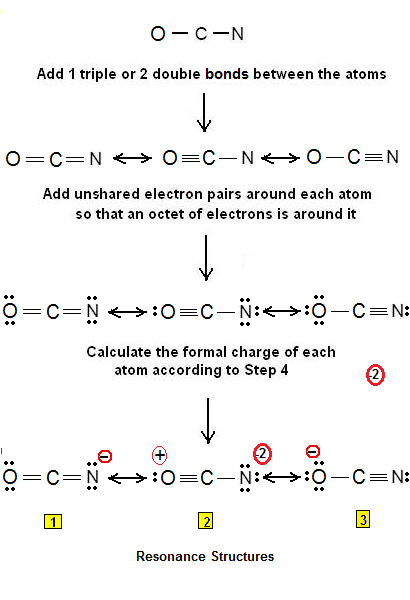
Structures #1 and #3 are more stable due to less charge separation comparing to #2. Structure #3 is more plausible than #1 since the more electronegative O atom has the negative charge. Therefore stability decreases: #3 > #1 > #2
The most plausible Lewis structure is #3 and the least plausible #2.
Relevant Posts
Lewis Structures|Octet Rule: A Simple Method to write Lewis Structures
Lewis Dot Structure of thiocyanate (SCN-)
Lewis Structures - How to draw Lewis Structure - NCN-2
References
- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)
Key Terms
resonance structures of cyanate ion OCN-, Lewis electron structures of cyanate ion, chemical formula of cyanate ion, simple procedure for drawing Lewis structures of cyanate ion,
This is awesome. We were just working this Lewis structure in our Chemistry 1 course and had a discussion as to why the triple bond-N form is the most likely structure. Thank you for devoting a discussion to it!
ReplyDelete