Lewis dot structures - Electron dot structure of CO
A simple method for writing Lewis Electron Dot Structures (Dot Electron Structures) was given in a previous article entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Let us consider the case of the Lewis electron dot structures of carbon monoxide CO. Carbon monoxide is an odorless, colorless, non-irritant gas. It is the most common cause of fatal poisoning in Britain today. It causes the accidental deaths of up to 50 persons each year in the U.K. alone and a much larger number of non-fatal poisonings.
Step 1: Connect the atoms with single bonds.

Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1):
Where n in this case is 2. Where V = (4 + 6) = 10 , V is the number of valence electrons of the carbon monoxide molecule.
Therefore, P = 6n + 2 – V = 6 * 2 + 2 – 10 = 4 So, there are either 2 double bonds or a triple bond.
Since the molecule has only two atoms there is no possibility for 2 double bonds.
Step 3 & 4: The dot resonance structures of CO (Lewis structures of carbon monoxide CO) are as follows:
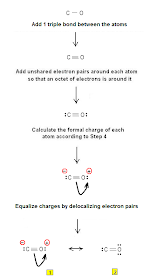
Lewis dot structure #1 is more stable since the octet rule is obeyed.
Relevant Posts
Simple Procedure for writing Lewis Structures- CO2, NCO
Lewis Dot Structure of Carbonic Acid H2CO3
References
- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)
Key Terms
simple method for drawing Lewis structures, Lewis structures of CO, Lewis electron dot structures of CO, carbon monoxide Lewis dot structures CO,
C has only 6 in the outermost shell
ReplyDeleteThank you! You are right. Structure #1 is more stable since the octet rule is obeyed. Structure #2 is less plausible since the C atom has only 6 outermost shell electrons!
Delete