Headspace
techniques
are employed in conjunction with gas chromatography (GC) analysis for certain type of samples. The GC can
handle any gaseous sample and any liquid sample that can be vaporized
completely and instantaneously before it goes to a proper column for
separation. Unfortunately there are many liquid samples (biological,
environmental) that cannot be directly injected into the GC. For such samples headspace analysis can be
used.
The principle
underlying GC headspace analysis is that in a sealed vial at constant
temperature equilibrium is established between volatile components of a liquid
or solid sample in the vial and the gas phase above it – the “headspace” (Fig.
I1). After allowing due time for equilibration (normally 15 min.) a portion of
the headspace – ambient volume above a sample matrix where the volatile compounds
exist in gaseous form at predictable levels
- may be withdrawn via a rubber septum using a gas-tight syringe and injected
into the GC column.
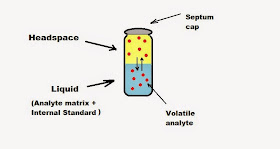 |
| Fig. I1: GC headspace
vial |
Headspace
analysis
is useful for situations where:
- The analyte of interest is volatile at temperatures below 290 ∘C
- The sample matrix is a solid, liquid, paste that is not easy to inject into a GC inlet
- Sample preparation to allow easy liquid injection is difficult
The following video demonstrates the headspace sampler
of a GC-FID system:
Advantages of Headspace Analysis
Headspace
analysis provides
several advantages over normal injections:
- Simpler sample preparation
- Directly analyze a wide range of sample matrices such as liquids, solids and pastes
- Columns last longer, with less maintenance. The headspace volume above the sample matrix is more clean than the matrix. By injecting fewer contaminants the analytical column lasts longer.
- High precision
- Solvent peak is smaller or nonexistent compared to traditional liquid injection GC techniques.
An internal
standard may be added prior to the heating process, and quantitative analyses
may be performed after constructing a calibration graph.
This technique is
widely used in the analysis of ethanol in blood (blood-alcohol in driving under influence (DUI) and driving whil and other volatile substances in
biological samples and in the pharmaceutical industry for measuring solvent
residues in tablets, amongst other applications. A gas chromatogram of an
in-house reference material containing ethanol, methanol and n-propanol
obtained by using headspace analysis is shown in Fig. I2.
The equations describing headspace theory derive from three physical laws associated with vapor pressure, partial pressures , and the relationship between vapor pressure of an analyte above a solution and the concentration of that analyte in the solution. These laws are: Dalton’s law, Henry’s law and Raoult’s law
“Applied Headspace Gas Chromatography”, B. Kolb Editions, Heyden, London, 1980
Theory of Headspace Analysis
The equations describing headspace theory derive from three physical laws associated with vapor pressure, partial pressures , and the relationship between vapor pressure of an analyte above a solution and the concentration of that analyte in the solution. These laws are: Dalton’s law, Henry’s law and Raoult’s law
According
to the above laws the basic principle involved in headspace theory is the
establishment of equilibrium between the sample phase and the headspace above
the sample. In a vial, at the end of the equilibration process, the concentration
of sample analyte in the headspace volume is given by the mass balance:
CO * VL = CG
* VG + CL * VL (1)
Where:
CO is the
concentration of analyte in the original sample (mol/ml)
CG is the
equilibrium concentration of analyte in the headspace (mol/cm3)
CL
is the equilibrium concentration of analyte in the liquid phase (mol/ml)
VL is the
volume of the sample (cm3)
VG is the
volume of gas in the sample vial (cm3)
Since the
distribution coefficient (Henry’s law constant) is defined as:
H = CL / CG (2)
By combining equations (1) and (2) and
rearranging:
1/ CG = (H/ CO) + (1/ CO) * (VG
/ CL) (3)
In most
cases at a low concentration range, the GC area count is directly proportional
to the gas phase concentration (CG).
Equation (3) can be rewritten as:
1/ A = (H*Rf/ CO) + (Rf /
CO) * (VG / CL) (4)
where A is
the GC area count and Rf is the GC response factor.
A plot of
the inverse of the GC area count (1/A) versus (VG / CL)
results in a straight line, as predicted by equation (4). The original
concentration, CO, can be obtained from the slope of the straight
line, while the dimensionless Henry’s law constant (H) can be calculated as the ratio of intercept to
slope.
References
“Applied Headspace Gas Chromatography”, B. Kolb Editions, Heyden, London, 1980
M. Markelov et al., Anal. Chim. Acta, 276, 235-45,
(1993)
T. Podar, J. of Chrom. Science, 35, 565 – 567, (1997)
R. J. Flanagan et al. “Fundamentals of Analytical
Toxicology”, John Wiley & Sons Ltd., 2007

Can you please provide a method for ethanol detection in blood ? We are using agilent hs gc ..Which perameters we have to use and how much amount of blood and internal standard add in vial ?. How calibration graph perform
ReplyDelete