The pH of a solution can be measured as follows:
What kind of substances are acid-base indicators?
Acid-base indicators are usually weak organic acids or weak organic bases. They tend to have different color depending on the pH of the solution in which they are in.
How acid-base indicators are prepared in the lab?
These are usually solid substances that are dissoved in a solvent (i.e. ethanol). Few drops of the solution of the indicator is added to the solution that we would like to determine the pH.
How simple acid-base indicators work?
In reality what happens is that the two forms of the indicator participate in an equilibrium:
ΗMeo + H2O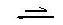 Meo- + H3O+ [1]
Meo- + H3O+ [1]
where ka is the ionization constant of methyl orange.
As a conclusion for monoprotic indicators of the general structure ΗIn with an equilibrium constant ka:
- By Acid-Base Indicators (less precise)
- By a pH-meter
What kind of substances are acid-base indicators?
Acid-base indicators are usually weak organic acids or weak organic bases. They tend to have different color depending on the pH of the solution in which they are in.
How acid-base indicators are prepared in the lab?
These are usually solid substances that are dissoved in a solvent (i.e. ethanol). Few drops of the solution of the indicator is added to the solution that we would like to determine the pH.
How simple acid-base indicators work?
An acid - base indicator is a colored substance that itself can exist in either an acid or base form. The acid form has a different color than the base form. Thus, the indicator turns one color in an acidic solution and another color if placed in a basic solution. If you know the pH at which the indicator turns from one form to the other, you can determine whether a solution has a higher or lower pH than this value.
For example methyl orange is one of the indicators commonly used in titrations. It gradually changes color from red to yellow over the pH interval from 3.1-4.4. In a solution with a pH > 4.4 exists as a species with negative charge (anion, Meo- ) and has a yellow color. In a solution with a pH < 3.1 exists in its neutral form and haw a red color (ΗMeo).
In reality what happens is that the two forms of the indicator participate in an equilibrium:
ΗMeo + H2O
If acid is added the position of the above equilibrium shifts to the left according to Le Chatelier's Principle and turns the indicator red (the solution takes a red color).
If base is added the position of the equilibrium shifts to the right according to Le Chatelier's Principle and turns the indicator yellow (the solution takes a yellow color).
The Ηenderson-Hasselbach equation can be used in order to determine the pH range an indicator changes color. Let's apply this for the methyl orange case:
If base is added the position of the equilibrium shifts to the right according to Le Chatelier's Principle and turns the indicator yellow (the solution takes a yellow color).
The Ηenderson-Hasselbach equation can be used in order to determine the pH range an indicator changes color. Let's apply this for the methyl orange case:
pH = pka + log [Meo-] / [ΗMeo] [2]
It has been determined experimentally that when 90% or more of the indicator is in the ΗMeo form (that means when the ratio [Meo-] / [ΗMeo] ≈ 0,1) then the color of the solution is red. If 90% or more of the indicator is in the Meo- form (that means [Meo-] / [ΗMeo] ≈ 10) the the color of the solution becomes yellow. By subsituting the above ratios to the Ηenderson-Hasselbach equation the pH range an indicator changes color can be determined:
pH = pka + log [Meo-] / [ΗMeo] = pka + log(0,1) = pka – 1 [3]
και
pH = pka + [Meo-] / [ΗMeo] = pka + log(10) = pka + 1 [4]
When [Meo-] = [ΗMeo] the color of the indicator is a mixture of yellow and red and the solution takes an orange color.
From equation [3] and [4 ] it can be determined that the indicator changes color over a range of two pH units (when the pH is between pka + 1 and pka - 1).
If pH < pka – 1, then the color of the solution takes the color of the ΗI form (unionized form)
If pH > pka + 1, then the color of the solution takes the color of the I- (ionized form)
If pH = pka then the color of the solution is a «mixture» of the colors of ΗI and I-.
In the video below the color change of an indicator is shown as the pH of the solution in which is in changes from neutral (pH of distilled water) to basic and then back from basic to acidic:
If pH > pka + 1, then the color of the solution takes the color of the I- (ionized form)
If pH = pka then the color of the solution is a «mixture» of the colors of ΗI and I-.
In the video below the color change of an indicator is shown as the pH of the solution in which is in changes from neutral (pH of distilled water) to basic and then back from basic to acidic:
We also provide analytical services and laboratory services to our customers. C.I. Acid blue 112
ReplyDelete