Lewis Electron Dot Structure of nitric acid HNO3
A simple procedure for writing Lewis Structures was given in a previous article entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
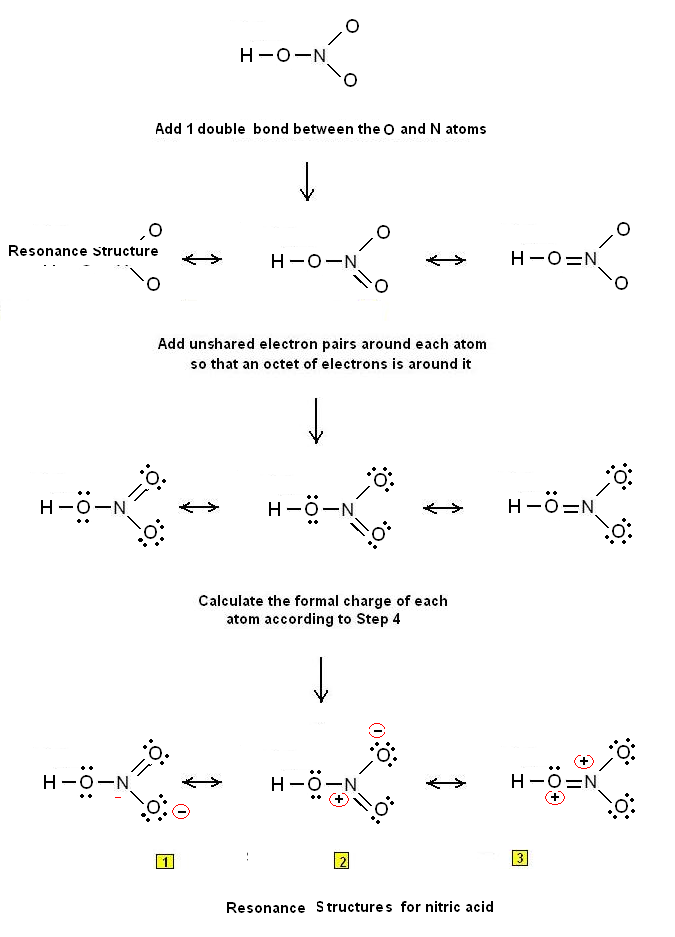
Let us consider the case of the Lewis electron dot structure of nitric acid HNO3 (HNO3 Lewis structure).
Nitric acid is a strong oxidizing agent and it dissolves practically all metals except gold and platinum and some other precious metals. As such, is an important raw material for the chemical and pharmaceutical industry. It is mainly used for etching and for the production of pure nitrates. Even though nitric acid was known since the 9th century - alchemists used it to separate gold and silver - its mass production started in 1902 when a German chemist Wilhelm Ostwald developed an industrial process. The German corporation BASF start producing it in an industrial scale by 1915. Initially it was used for the production of explosives but today its main use is for the production of fertilizers such as ammonium nitrate. Other main applications is for the production of explosives, nylon precursors and substituted organic compounds.
In elemental analysis by atomic absorption spectroscopy, ICP, graphite furnace atomic spectroscopy dilute nitric acid is used as a "solvent" for the determination of metal traces in solution.
Let us draw the Lewis dot structure of nitric acid :
Step 1: The central atom will be the N atom since it is the less electronegative (H is a terminal atom – it cannot be a central atom). Connect the atoms with single bonds: