Lewis Structures of the Phosphate Ion (PO4)-3
A simple method for writing Lewis electron dot structures is given in a previous article entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Another example for writing Lewis structures following the above procedure is given below.
Let us consider the case of the Lewis structures of phosphate ion PO4-3 . The phosphate ion combines with various atoms and molecules withing living organisms to form many different compounds essential to life. Some examples of phosphate role in living matter include:
- Giving shape to DNA, which is a blueprint of genetic information contained in every living cell
- Playing a vital role in the way living matter provides energy for biochemical reactions in cells (ATP, ADP, AMP). The compound adenosine phosphate (ATP) stores energy living matter gets from food (and sunlight in plants) and releases it when it is required for cellular activities
- The forming and strengthening of bone and teeth
Humans and animals get phosphate from the food they eat. Plants get phosphate from the soil. Fertilizer is added to phosphate-deficient soil to replenish this vital chemical species.
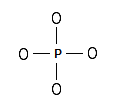
Step 1: The central atom will be the P atom since it is the less electronegative. Connect the atoms with single bonds:
Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1) in the article entitled “Lewis Structures and the Octet Rule”.:
Where n in this case is 5 since PO4-3 consists of five atoms. Where V = (5 + 6 + 6 + 6 +6 ) - (-3) = 32
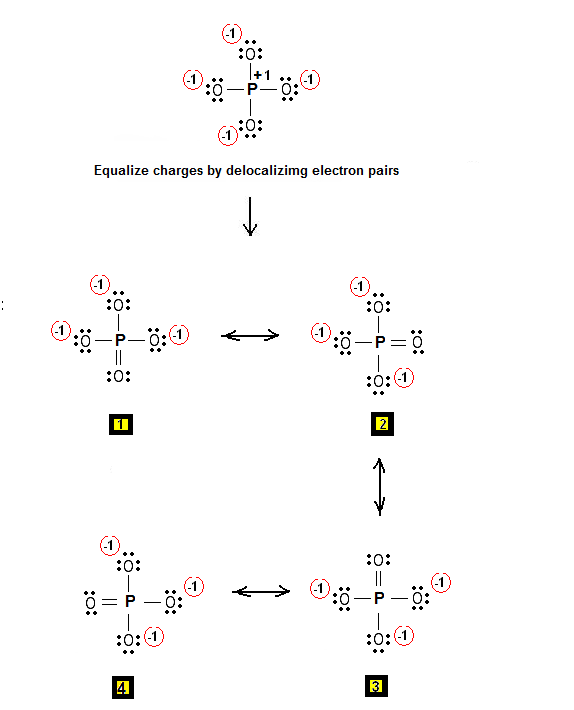
Therefore, P = 6n + 2 – V = 6 * 5 + 2 – 32 = 0 So there are no π electrons in PO4-3
Step 3 & 4: Therefore, the Lewis structures for PO4-3 are as follows:
Relevant Posts
Lewis Structures|Octet Rule: A Simple Method to write Lewis Structures
Lewis structure of PO3-1 – Simple Procedure for Dot structures
References
- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)
- Steven S. Zumdahl, “Chemical Principles” 6th Edition, Houghton Mifflin Company, 2009
Key Terms
Lewis structures of, simple method for writing Lewis electron dot structures, Lewis electron dot structures, electron dot structures



