Simple Method for writing Lewis Structures – Electron Dot Structures of Trifluoronitrosomethane CF3NO
A simple procedure for writing electron dot structures (Lewis structures) is given in a previous article entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Another example for writing Lewis structures following the above procedure is given below.
Let us consider the case of of trifluoronitrosomethane (CF3NO). It is used as a monomer (building block) for specialty rubbers (nitroso rubber in conjuction with tetrafluoroethylene) and as a military poison gas.
Let us draw the CF3NO electron dot structures?
Step 1: The central atom will be the C atom since it is the less electronegative. Connect the atoms with single bonds:

Step 2:
Calculate the # of electrons in π bonds (multiple bonds) using formula (1) in the article entitled “Lewis Structures and the Octet Rule”.
Where n in this case is 6 since CF3NO consists of six atoms.
Where V = (7*3 + 4 + 5 + 6 ) = 36
Therefore, P = 6n + 2 – V = 6 * 6 + 2 – 36 = 2
So, there are 2 π electrons in CF3NO
Therefore, 1 double bond must be added to the structure of Step 1.
Step 3 & 4: The Lewis electron dot structures for CF3NO are as follows:
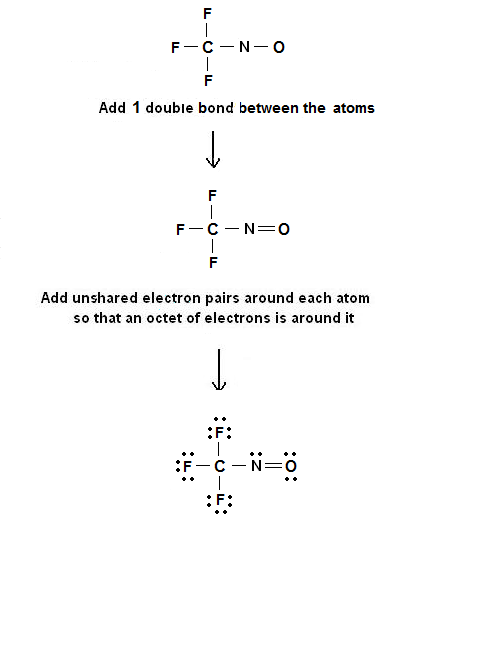
The only position that the double bond can be placed is between the N and O atom.
Relevant Posts - Relevant Videos
Lewis Structures|Octet Rule: A Simple Method to write Lewis Structures
A Brief Tutorial on drawing Lewis Structures of the N2F+
Lewis Structures of peroxy nitric acid HO2NO2
References
- G.N. Lewis, J.A.C.S, 38, 762-785 (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821 (1972)
Key Terms
resonance structures of CF3NO3 , Lewis electron structures of , chemical formula of CF3NO3, simple procedure for drawing Lewis structures of nitric acid,