Rules for writing Lewis electron resonance structures– Formamidine
A simple and automatic method for writing Lewis Dot Structures is given in a previous post entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Let us consider the case of the Lewis Dot Structures of formamidine HNCHNH2
Step 1: Connect the atoms with single bonds:
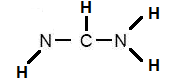
Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1):
Where n in this case is 3 since formamidine has 3 non-hydrogen atoms.
Where V = 1*1 + 1*5 + 1*4 + 1*1 +1*5 +1*1 +1*1 = 18, V is the number of valence electrons of the atom.
Therefore, P = 6n + 2 – V = 6 *3 + 2 – 18 = 2 there is 1 double bond.
The Lewis electron resonance structures of formamidine are as follows:

Structure #1 is the most stable electron dot structure because there is an octet of electrons around each atom and there is no charge separation.
Relevant Posts
Lewis Structures and the Octet Rule - A simple method to write Lewis Structures
Simple Method for writing the Lewis Structures of carbon disulfide CS2
References
- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)
No comments:
Post a Comment