Lewis Electron Dot Structure of dichromate Cr2O7-2
A simple procedure for writing Lewis electron dot structures is given in a previous article entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Another example for writing Lewis structures following the above procedure is given below.
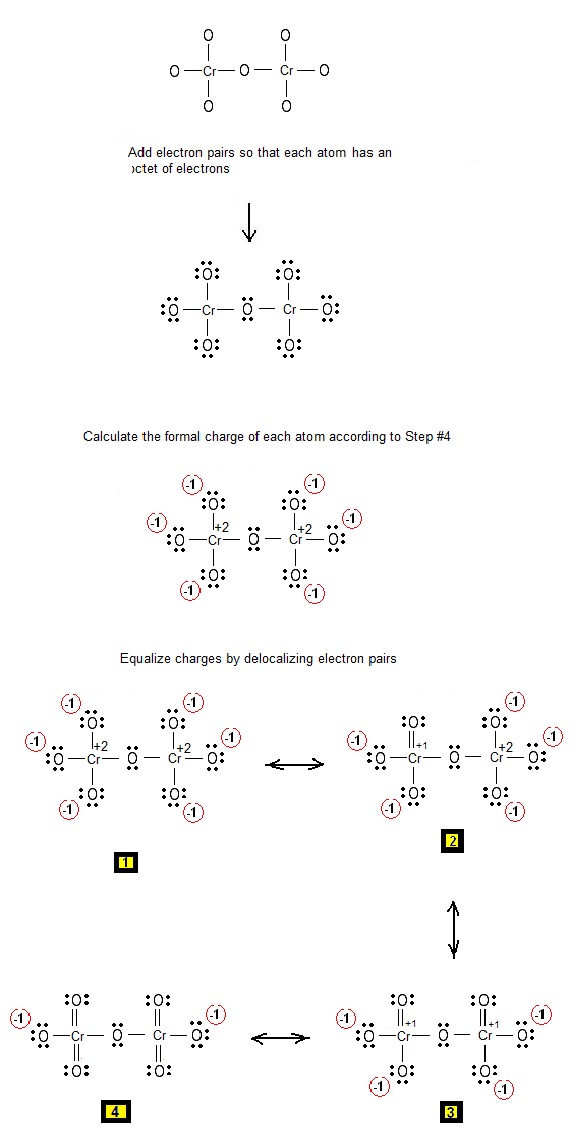
Let us consider the case of the Lewis electron dot structures of the dichromate anion Cr2O7-2. Consider that there is a Cr-O-Cr bond.
Chromates and dichromates are used in chrome plating to protect metals from corrosion and to improve paint adhesion. Chromate and dichromate salts of heavy metals and alkaline earth metals are only very slight soluble in water and are thus used as pigments. Dichromate is converted to chromic sulfate for tanning leather. The reaction of chromium with collagen raises the hydrothermal stability of the leather and makes it resistant to bacterial attack. The reaction with collagen is also a useful reaction in photography as a sensitizer for gelatin coatings.
How can we draw the Lewis structures of Cr2O7-2 ?
Step 1: Connect the atoms with single bonds.

Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1) in the article entitled “Lewis Structures and the Octet Rule”.
Where n in this case is 9. Where V = (6 + 6 + 6 + 6 + 6 + 6 + 6 + 6 + 6 ) – (-2) = 56 , V is the number of valence electrons of the dichromate ion.
Therefore, P = 6n + 2 – V = 6 * 9 + 2 – 56 = 0
So, there are no multiple bonds in the molecule.
So the structure of Step 1 is the Lewis structure.
Electrons are placed around each atom so that charge is minimized.
Step 3 & 4: The Lewis resonance structures of dichromate Cr2O7-2 are as follows: