Electron Dot Structures of peroxy nitric acid HO2NO2
A simple procedure for writing electron dot structures (Lewis structures) is given in a previous article entitled “Lewis Structures and the Octet Rule”. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Another example for writing Lewis structures following the above procedure is given below.
Let us consider the case of of peroxy nitic acid (HO2NO2) (known also as PAN HNO4).
Peroxynitric acid HNO4 plays an important role in the coupling of atmospheric HOx and NOx cycles4, especially at low temperatures. PNA serves as an important HOx and NOx reservoir species altering the oxidative capacity of the atmosphere on regional and global scales5. PNA is formed via the reaction of HO2 and NO2.
Let us draw the HO2NO2 electron dot structures?
Step 1: Connect the atoms with single bonds.
The central atom will be the N atom since it is the less electronegative (H is a terminal atom – it cannot be a central atom).
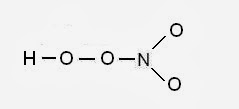
Step 2:
Calculate the # of electrons in π bonds (multiple bonds) using formula (1) in the article entitled “Lewis Structures and the Octet Rule”.
Where n in this case is 5 since HO2NO2 consists of six atoms but one of them is a H atom.
Where V = (1 + 6 + 6 + 5 + 6 + 6) = 30
Therefore, there are 2 π electrons in HO2NO2 and so 1 double bond must be added to the structure of Step 1.
Step 3 & 4: One double bond must therefore be placed through the 3 N-O bonds. Therefore, the Lewis electron dot structures for HO2NO2 are as follows:

Stuctures #1 and #2 are the more plausible (more stable) due to smaller charge separation.
Relevant Posts - Relevant Videos
Lewis Structures|Octet Rule: A Simple Method to write Lewis Structures
Simple Method for writing Lewis Structures – Lewis Structures of CF3NO
References
- G.N. Lewis, J.A.C.S, 38, 762-785 (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821 (1972)
- H. Niki et al., Chem. Phys. Lett., 45, 564-566 (1977)
- S. Kim et al., J. Geophys. Res.-Atmos., 112, D12S01 (2007)
Key Terms
resonance structures of HO2NO2 peroxy nitric acid , Lewis electron structures of , chemical formula of HO2NO2 peroxy nitric acid, simple procedure for drawing Lewis structures of PNA peroxy nitric acid,