In normal-phase chromatography a polar stationary phase is used in
conjunction with a less polar mobile phase for elution of the analytes (in contrast to reversed phase chromatography where a nonpolar stationary phase is used with a more polar mobile phase). Neutral
solutes in the mobile phase are separated on the basis of their polarity. The
more polar the solute the more it is
retained on the stationary phase. The mobile phase that it is used is normally
less polar than the stationary phase. If the polarity of the mobile phase
continuously increases (in a gradient elution scheme) the solute retention is
decreased.
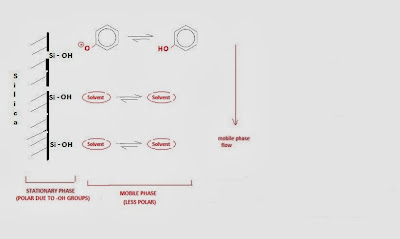
The stationary phases used in normal phase chromatography are
usually silica or alumina and they are polar as a result of hydroxyl groups
(-OH) (Fig. 1). The surface hydroxyl groups interact with the functional groups on the
solute molecules and depending on the strength of this interaction
preferentially absorb one solute relative to another (absorb the more polar
compound relative to the less polar). In case the solute is a neutral molecule
that has a permanent dipole or if a dipole can be induced on it, then it will
be attracted by dipole-dipole interaction to the stationary phase.
A mechanism that describes the adsorption process in normal phase
liquid chromatography is shown in Fig. 1.
Silica is the preferred stationary phase mainly because its
availability, known performance and low cost. For basic compounds such as
amines, which are very strongly retained on silica, alumina can be used as an
alternative. In addition to the above stationary phases, a variety of polar
bonded phases are used with functional groups such as cyano [-(CH2)3CN],
amino [-(CH2)nNH2], diol, bonded to the silica stationary
phase.
The mobile phases used in normal phase chromatography are nonpolar
hydrocarbons such as hexane, heptane, octane (Table I.1) to which is added a
small amount of a more polar solvent such as 2-propanol.
Solvent selectivity is controlled by the nature of the added
solvent. For example:
- solvents that are good proton donors such as water and chloroform interact preferentially with basic solutes such as amines
- solvents that are good proton acceptors such as alcohols, ethers and amines tent to interact best with acids and phenols
- solvents with large dipole moments such as methylene chloride interact preferentially with solutes that have large dipole moments such as nitriles, amines, sulfoxides and nitro compounds.
Mobile Phases used in Normal Phase Chromatography
|
|||
Solvent strength ε○
|
|||
Solvent
|
Silica
|
Alumina
|
Solvent Properties
|
n-hexane
|
0.01
|
0.01
|
nonpolar
|
n-heptane
|
0.01
|
0.01
|
nonpolar
|
isooctane
|
0.01
|
0.01
|
nonpolar
|
chloroform
|
0.26
|
0.40
|
proton acceptor
|
methylene chloride
|
0.32
|
0.42
|
large dipole
|
ethyl acetate
|
0.38
|
0.58
|
proton donor
|
THF
|
0.44
|
0.57
|
proton acceptor
|
propylamine
|
~0.5
|
-
|
proton acceptor
|
acetonitrile
|
0.5
|
0.65
|
dipole
|
methanol
|
~0.7
|
0.95
|
proton acceptor
|
Table I.1: Solvents that are
used as mobile phase in normal phase chromatography. The solvent strength
parameter ε○ defines the strength of the solvent. In normal phase chromatography a
solvent with a low ε○ is
chosen and quantities of a second solvent with a greater ε○ is added until the
desired separation is achieved.
In general, the order of elution for species separated by normal
phase chromatography is the following:
Saturated
hydrocarbons < olefins < aromatic hydrocarbons ≈ organic halides <
sulfides < ethers < nitro compounds < esters, aldehydes, ketones <
alcohols , amines < sulfones < amides < carboxylic acids.
The more polar compounds are retained longer than the nonpolar
compounds such as satutated hydrocarbons.
Normal phase chromatography is mainly applied to the analysis of
samples that are soluble in non-polar
solvents. Amongst them isomeric and multifunctional compounds are best
separated by this method. It is also possible to separate species on the basis
of the number of electronegative atoms such as oxygen or nitrogen. Fat and
water-soluble vitamins, pesticides and hydrocarbons have all been separated
using hexane as the mobile phase.
The major disadvantages of normal phase chromatography are the
following:- Lack of selectivity of the stationary phase (all compounds are eluted in the same order regardless of the stationary phase selected. The mobile phase is therefore used to achieve any change in selectivity).
- Absorption of water (water absorbed from the atmosphere is adsorbed onto the strongest adsorption sites on the stationary phase leading to a decrease in solute retention – deactivation of the stationary phase).
References
1) L.R. Snyder. J.J. Kirkland, “Introduction to Modern Liquid
Chromatography”, 2nd edition, Wiley, 1979
2) A. Weston and P. Brown, “HPLC and CE Principles and Practice”,
Academic Press, 1997
3) A. Braithwaite and F.J. Smith, “Chromatographic Methods”, 4th
Ed., Chapman & Hall, 1990
No comments:
Post a Comment