Sugar Caramelization Chemistry - Caramelization in Cooking
Foods with high carbohydrate and low nitrogen content can be caramelized when heated. It is well known from experience that flavor develops when a food is cooked (heated). There are a number of dishes (such as the cre´me brulees) where the flavors and color of the caramelization reactions are desirable. The most important reactions, from the prespective of flavor and aroma development, are those that are thermally activated. Amongst these reactions, the Maillard, Strecker and caramelization reactions are largely responsible for the flavors of chocolate and coffee, the caramel flavors of cooked sugars in deserts, on the crust of freshly baked bread and on the characteristic flavors of cooked meats.
The process of caramelization starts with the melting of sugar at temperatures above 120 °C. The main reactions that occur during the heating process are the following:
- Sugars are dehydrated and double bonds are introduced into the structures.

Fig. I.1: Caramelization - Brown colored products with a typical caramel aroma are obtained by melting sugars (glucose in this case). A large number of furan and pyran compounds are formed.
A large number of furan (have a nutty aroma) and pyran compounds are produced (Fig. I.1). The formation of these compounds can be explained by enolizations and dehydrating reactions of carbohydrates (mechanisms are shown in Fig. I.2a and I.2b). The reaction pathway in acidic conditions starts slowly with enolization to key intermediates called enediols. Glucose, for example, reacts to form 1,2-enediol and fructose gives rise to 2,3-enediol. These enediols further react to produce 5-hydroxymethyl furfural (HMF) and 2-hydroxyacetyl furan respectively. The steps for the production of HMF from 1,2-enediol involves elimination of water (retro-Michael addition) at C-3 and subsequently at C-4 and cyclization of the intermediate produced to a hemiacetal which releases another water molecule to produce HMF (Fig. I.2a).

Fig. I.2a: Formation of HMF from the intermediate 1,2-Enediol after water elimination and subsequent cyclization
The steps for the production of 2-hydroxyacetyl furan from 2,3-enediol (produced from fructose) involve water elimination at C-4 followed by a second water eleimination at C-5. (Fig. I.2b). With 2,3-enediol another reaction pathway is also possible the elimination of hydroxyl group at C-1 producing a dihydropyranone - 3,5-dihydroxy-2-methyl-5,6-dihydropyran-4-one - after cyclization (Fig. I.2b). It is worth to mention that both HMF and 3,5-dihydroxy-2-methyl-5,6-dihydropyran-4-one are used as indicators for the heating of foods containing carbohydrates (i.e honey).
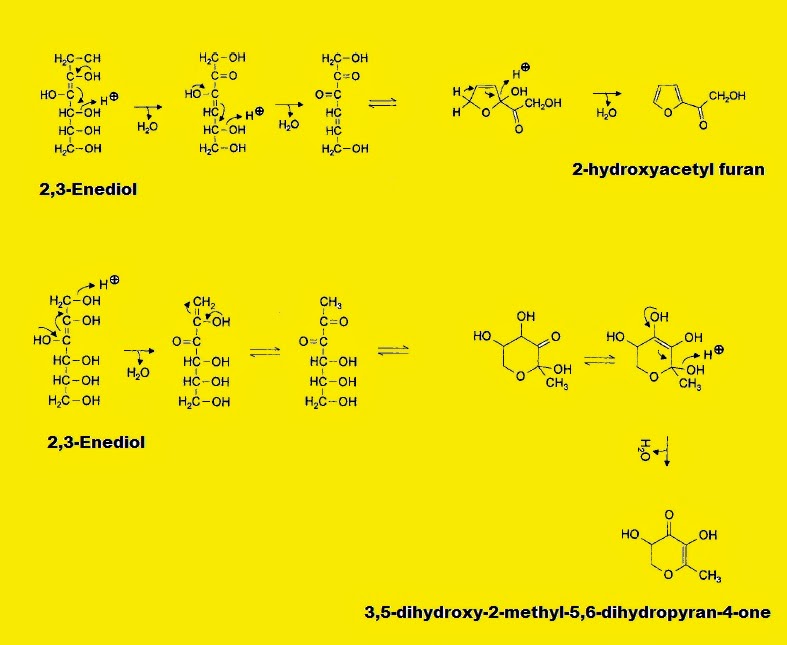
Fig. I.2b: Mechanism of formation of 2-hydroxyacetyl furan and 3,5-dihydroxy-2-methyl-5,6-dihydropyran-4-one from the intermediate 2,3-Enediol after water elimination and subsequent cyclization
- The small sugar molecules react together by condensation reactions (at temperatures above 150 °C) to produce polymers with conjugated double bonds which absorb light and give brown colors
Heating for example glucose syrup with sulfuric acid in the presence of ammonia produces intensively colored polymers ("sucre couleur"). Caramelization of sucrose (table sugar) requires a temperature of approximatelly 200 °C. At 160 °C, sucrose melts and forms glucose and fructose anhydride. At 200 °C, the reaction process consists of three distinct stages well separated in time. The first step requires 35 minutes of heating and a weight loss of 4.5 % is observed in the solution, corresponding to a loss of one molecule of water per molecule of sucrose. After an additional 55 minutes of heating, the weight loss amounts to 9% and the pigment formed is named caramelan (Fig I.2c). Caramelan is soluble in water and ethanol and has a bitter taste. A further 55 minutes of heating leads to the formation of caramelen. This compound corresponds to a weight loss of about 14%, which is about eight molecules of water from three molecules of sucrose (Fig I.2c). Caramelen is soluble in water and melts at 154 °C. Additional heating gives a very dark, nearly insoluble pigment C125H188O80 called caramelin or humin. The typical caramel flavor is the result of a number of sugar fragmentation and dehydration products like diacetyl, acetic acid, formic acid and furanone.

Fig. I.2c: Caramelization of sucrose (table sugar) leading to the formation of caramelan and caramelen
- Smaller volatile compounds are formed by fragmentation reactions of sugars and these give unique flavors and fragrances (Fig. I.3)
Hydroxyaldehydes and hydroxyketones are formed by chain cleavage of sugars (fructose in Fig. I.3) due to retroaldol reactions in the presence of dilute alkali at high temperatures. Fructose can yield glyceraldehyde and dihydroxyacetone which easily undergoes water elimination to produce 2-oxopropanal ( pyruvaldehyde) used often as a synthetic flavoring agent.

Fig. I.3: Mechanism of formation of glyceraldehyde and pyruvaldehyde from fructose during the caramelization process (heating above 120 °C)
In the presense of amines reactions like the above take place under milder conditions (Maillard reaction) and in addition serve as a source for other heterocyclic compounds to be generated. The intermediate stage of the Maillard reaction and related reactions provides a complex pool of reactive compounds, which are subjected to rearrangements and further reactions producing several classes of heterocyclic (volatile) products, several of which are important for cooked flavor.
Relevant Posts
Food Chemistry: Chemical reactions in cooking
Food Chemistry: Antioxidants and Oxidation Reactions in Foods
References
- P. Barham et al., Chem. Rev., 110, 2313 (2010)
- P. Walstra, "Physical Chemistry of Foods", Marcel Dekker Inc., 2003
- J.M DeMan, "Principles of Food Chemistry", 3rd Edition, Aspen Publishers Inc., 1999
- H-D. Belitz et al., “Food Chemistry”, 4th Edition, Springer Verlag, 2009
Key Terms
caramelization, caramelization reactions, caramelization of sucrose, enolization, , carbohydrates, HMF, caramel flavors
Thanks for sharing informative article related to Caramelization in Cooking and appreciate that the content is very well supported by chemical and molecular structures. Sharing following website which will add value to the content and will help people who are looking related to Caramelization.
ReplyDeletehttp://www.scienceofcooking.com/caramelization.htm
http://bakerpedia.com/processes/caramelization/
http://prospect.rsc.org/blogs/cw/2012/11/13/food-chemistry-carnival-the-sweet-gooey-world-of-caramel/
http://www.dyespigments.net/
Great
ReplyDelete