Analytical Chemistry is the branch of chemistry concerned with determining the qualitative and quantitative composition of substances. It is a branch which is broad in scope and its applications extend to all parts of an industrialized society.
The two main sub-branches of Analytical Chemistry are:
- Qualitative analysis: The determination of the identity of chemical species present in a sample
- Quantitative analysis: An examination to determine how much of a particular species is present in a sample
The procedure followed while doing a chemical analysis can be classified as either a classical (“wet”) procedure or an instrumental procedure. Therefore, both qualitative and quantitative analysis are sub-divided into classical (“wet”) analysis and instrumental analysis.
In wet procedures chemical reactions are used to perform the analysis and there is no use of any mechanical or electronic instrument except of an analytical balance. Main sub-branches of classical ("wet") analysis are:
- Gravimetric analysis, or quantitative estimation by weight, is the process of isolating and weighting an element or a compound of the element in as pure form as possible. The main object in gravimetric analysis is the transformation of the element or radical into a stable, pure compound which can be readily converted into a form suitable for weighting. The weight of the element is calculated from the formula of the compound and atomic weights of the elements that are constituents of the compound. The separation of the element or its compound may be accomplished by precipitation methods, volatilization or electroanalytical methods
- Volumetric (titrimetric) analysis, is the analysis in which we measure the volume of a reagent reacting stoichiometrically with the analyte. It first appeared as an analytical method in the early eighteenth century and initially did not receive wide acceptance. The growth and acceptance of volumetric methods required a deeper understanding of stoichiometry, thermodynamics and chemical equilibria. By the early 20th century the accuracy and precision of volumetric methods were comparable to that of gravimetric methods, establishing an accepted analytical technique.
Titrimetric methods are classified into four categories based on the type of reaction involved: Acid-base, complexometric, redox and precipitation titrations.
In instrumental procedures there is use of instruments of some sort either to make a critical measurement or to perform the entire analysis. Modern day analytical chemistry is performed with the aid of instruments such as: NMR Spectrometer, Mass Spectrometer, I.R., FT-I.R, Gas Chromatograph or Liquid Chromatograph, ICP, Craphite Furnace Atomic Absorption Spectrometer, Atomic Absorption Spectrometer, XRF, Cyclic Voltametry.
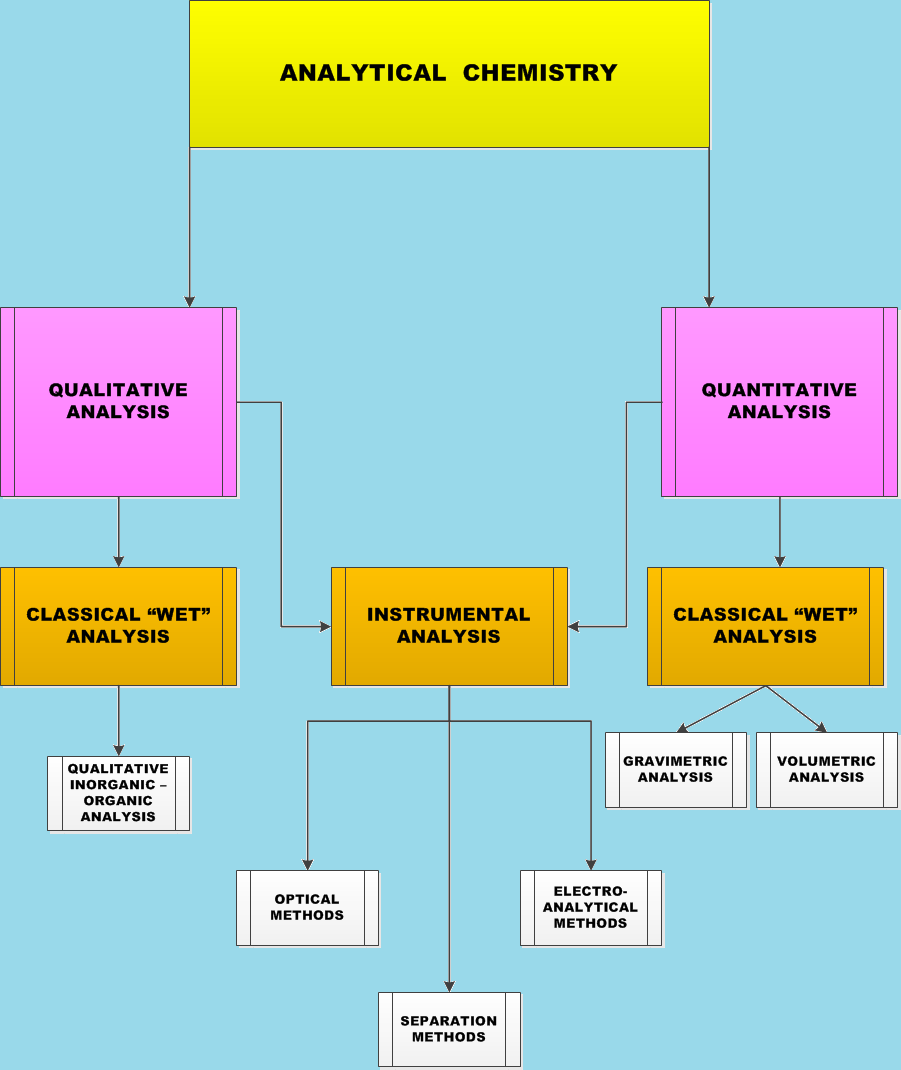
Optical methods of analysis are also called spectroscopic methods. The first instruments were developed for use in the visible region and therefore the methods were called optical methods. All spectroscopic methods are based on the interaction of electromagnetic radiation with the quantized energy states of the matter. By these methods, we study the measurement of a quantity based on emission, absorption, scattering or change in some property of electromagnetic radiation depending on the nature or the amount of the constituent on the sample. These methods are classified based on either the type of effect (emission, absorption or scattering) or the type of the electromagnetic radiation (IR, visible, x-ray). The most important spectroscopic methods are given below:
- Atomic Absorption Spectroscopy – Flame Photometry – Atomic Fluorescence Sprctroscopy
- Emission Specroscopy
- Raman Spectroscopy
- Microwave Spectroscopy
- U.V. Absorption Spectrophotometry
- Infrared Spectrophotometry
- Fluorophotometry - Phosphorimetry
- Turbidimetry – Nephelometry
- Refractometry - Interferometry
- Raman Spectroscopy
- X-ray: Absorption, Emission, Diffraction
- Nuclear Magnetic Resonance Spectroscopy – Electron Spin Resonance Spectroscopy
- Gamma-ray Spectroscopy – Mossbauer Spectroscopy
- D. Harvey, “Modern Analytical Chemistry”, McGraw-Hill Companies Inc., 2000
- R.D. Brown, “Introduction to Chemical Analysis”, McGraw-Hill Companies Inc., 1982
- S.M. Khopkar, “Basic Concepts of Analytical Chemistry” , New Age Int. Ltd. Publishers, 2nd, 1998
Wow, great post.
ReplyDeleteIndeed!
DeleteIf you want to know more about analytical chemistry, visit our site https://zodiaclifesciences.com/by-hplc-column/ or you can visit our blog https://zodiac-hplc-columns.blogspot.com/
I just wanted to say thank you for such an insightful comment! It is really helpful, and exactly what I was looking for. The effort you make to clarify things and make them easy to understand is something that is truly appreciated. As a professional in this area, it's always great to find such content, especially when searching for a trusted chemistry lab equipment manufacturer in India chemistry lab equipment manufacturer in India. Well done!
ReplyDelete
ReplyDeleteThermal Analyzer
Great detailed information, I’ll be visiting you more frequently, here is very interesting information.
ReplyDeleteChemistry Services