A Simple and General Method to write Lewis Structures
Lewis structures constitute an overwhelming majority of the structures that students encounter in courses in general chemistry, organic chemistry, or biochemistry. Hence, the writing of Lewis structures corresponding to a given molecular formula is an important exercise for them. The ability of students to write these structures correctly and then to calculate the formal charges of the atoms involved, is a prerequisite to understanding such topics as:
· Reπactivity
· Isomerism and molecular geometry.
Since many chemistry textbooks do not give easy procedures for writing such
structures we present a set of instructions for writing Lewis structures
(1-3).
But at first let’s see the following:
Which are the essential propositions of the Lewis theory for bonding?
Chemical bonding is at the heart of understanding chemistry. In 1916,
G.N. Lewis published a paper on the chemical bond in nonionic substances,
entitled “The
Atom and the Molecule” ( ). The Bohr
theory of the atom, proposed a few years earlier, suggested the possibility of a
rational explanation for the patterns of chemical combination shown by the elements
that had earlier led to the periodic table. G.N. Lewis using essentially empirical
concepts along with his chemical intuition combined observations at the time about
chemical bonding. The theory he proposed not only was essential in understanding
how elements bonded but also provided a visual representation for them the so
called Lewis symbols and the Lewis dot structures.
Lewis symbols: A Lewis symbol consists of the element
symbol surrounded by dots to represent the number of electrons in the outer
shell. The number of electrons in the outer shell is correlated by simply reading
the group number.
The Lewis symbol for fluorine is shown below:
 |
|
Figure 1: Lewis symbol for the atom of fluorine. The
valence electrons are shown as dots around the chemical symbol of
fluorine
|
Lewis structures (or electron dot structures)
are a simplistic way of representing the electrons in molecules. Valence electrons
– electrons of the outermost orbital of the atom - are represented as
dots surrounding the symbol of an element
i.e. the Lewis structure for the ion of CN-
i.e. the Lewis structure for the ion of CN-
The essential propositions of the Lewis theory for bonding were based on the fact
that atoms with 8 electrons in their outer shell (like the noble gases) exhibit
increased stability ( octet rule ).
· Chemical bonds are
formed when atoms transfer valence electrons to attain noble gas electron
configutations ( octet of electrons in the outermost orbital, duet of electrons in
the case of hydrogen). The type of bond formed in this case is called an ionic
bond.
· Chemical bonds are formed when atoms share valence electrons to attain noble
gas electron configurations . The type of bond formed in this case is called a
covalent bond.
Which are the types of bonds proposed by Lewis?
There are two basic types of bonding proposed in the Lewis theory:
· Ionic bonding: This type of bonding occurs when outer
electron(s) are transferred from one atom to an other so that both of them attain
an octet of electrons in their outermost shell. The two atoms are held together by
electrostatic forces
Ionic bonding occurs between metals and non-metals. The metal gives its
outer electrons (it gains a positive charge since it looses electrons) to the
non-metal (it gains a negative charge). As the ions formed have opposite charges
they will attract each other and this attractive or electrostatic force is called
an ionic bond.
 |
| Figure 2: Schematic representation of the formation of an ionic bond by the transfer of an electron from the outer shell of the Na atom to the outer shell of the Cl. |
Please see the following video on the formation of ionic bonds:
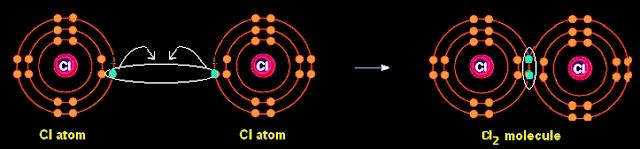
· Covalent bonding: This type of bonding forms when two non-metal atoms
share a pair of outer electrons so that both of them attain an octet of electrons
in their outermost shell.
Is there a simple procedure enabling one to write down correctly and rapidly Lewis structures for compounds formed by elements of the three first rows of the periodic table?
Compounds formed by elements of the first three rows of the periodic table
generally obey the Octet Rule . However, until expertise has been gained,
confusion often arises concerning:
· The correct number of multiple bonds to use
· The correct number of valence electrons to use
· The placing of formal charges
Therefore, it is not as straightforward as it seems when it comes to write
down a Lewis structure
A simple procedure for writing Lewis structures for
molecules that eliminates all the above problems is as follows (3):
1) Find the central atom of the molecule. Connect the central atom
with the other atoms of the molecule with single bonds.
The central atom usually has a “subscript” of 1 in the molecular
formula. If there are present two atoms with “subscript” 1 then the
central atom is the less electronegative of the two (for an electronegativity table
see here) .
The central atom can not be a hydrogen atom
i.e. Consider the molecule with a general molecular formula ABx.
Identify the central atom.
The central atom
is the A atom since it has a subscript equal to 1.
2) Calculate the number of electrons which must be shared through π
bonds (pi bonding), P in the molecule:
P = 6n + 2 –
V
(1)
Where n is the number of atoms in the molecule
excluding the hydrogen atoms
V is the total number of valence electrons in the molecule =
V is the total number of valence electrons in the molecule =
=
( sum of group numbers of the
atoms in the molecule) – charge
3) Add
double or triple bonds to the structure in Step 1 according to the results
obtained in Step 2. Add unshared pairs of electrons around each atom so that
all of them have octets around them (except for the H atom)
4) Calculate the formal charges of the atoms in the molecule as follows: i)
Any atom having its normal valency number of bonds will be electrically
neutral; any atom which does not have its normal valency number of bonds will
have a formal charge ii) The formal charge may be calculated by
subtracting the number of electrons “owned” by the element from its
group number
Formal Charge of Element = Group Number – Total number of electrons “owned”
by the atom concerned
(2)
As an example let us write the Lewis structure for the
CN- ion:
Step1: Since the molecule has 2 atoms and it is linear there is no central
atom. Connect the 2 atoms with a single bond.
Step 2: Calculate the # of electrons in p bonds (multiple bonds) using formula
(1):
Where n in this case is 2 since CN-
consists of two atoms
Where V = (4 + 5) – (-1) = 10
Therefore, P = 6 * 2 + 2 – 10 = 4 So there are 4 π (pi) electrons in CN- and that means either 2
double or 1 triple bond.
Since there are only 2 atoms there is no possibility for 2 double bonds
The 2 atoms are joined together with a triple bond. Therefore a draw for the
Lewis structure of CN- is as follows:
Additional examples on this subject are given in the article “Simple
Procedure for writing Lewis Structures – Examples”.
Links to worked examples can also be found on the Sitemap - Table of Contents (Lewis Electron Dot Structures)..
Links to worked examples can also be found on the Sitemap - Table of Contents (Lewis Electron Dot Structures)..
Relevant Posts - Relevant Videos
Lewis Electron Dot Structures
References
- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)
Key Terms
Lewis structures, how can I write
the Lewis structure, pi and, for the draw, Lewis symbols,
electron dot structures, ionic
bonding, covalent bonding, structure de lewis, representation de
Lewis, estructura de Lewis, 路易士结构, formule de Lewis



How can I use the above method in order to draw the Lewis structure of SO3?
ReplyDeleteThe above method was used to draw the Lewis electron dot structure of SO3. Please see the following post: "Lewis structures of SO3" http://chem-net.blogspot.com/2011/05/simple-procedure-for-writing-lewis_27.html
DeleteCan the above method be used to draw the Lewis structure of cyclic molecules?
ReplyDeleteClearly cyclic molecules can also be handled by this procedure by taking into account the fact that an n atom cyclic molecule will share 2n (rather 2(n-1)) σ electrons
ReplyDeleteCan I use the above method to draw Lewis structures in which more than eight electrons are around an atom?
ReplyDeleteLewis structures in which more than 8 electrons surround an atom are possible if this atom is that of an element found in the third row or a subsequent row of the periodic table. The simple method given above can be used to write Lewis structures with expanded octets. Please see the case of the sulfate ion:
Deletehttp://chem-net.blogspot.com/2013/02/electron-dot-structure-of-sulfate-ion.html
Many thanks for this excellent guide to drawing Lewis structures. Just one question, how did you arrive at the formula for calculating the number of pi electrons?
ReplyDeletePlease see reference 3 above.
DeleteThanks. I feel confident this article will be very useful in my up-coming exam.
ReplyDeleteRegards.
Pls can u explain benzene 's structure using this
ReplyDeleteIn PCL5 .. P (Pi Electrons) turns out to be -2 (6*6+2 - 5*7+5). What does this mean? How should one proceed in such situation?
ReplyDeleteSOCl2 how can we draw it ?
ReplyDeletethe total valence is 26 and the P=6n+2-v is also 26 which gives ZERO ..!!
please help and thanks
I have it as an example of an expanded octet. Please check at: http://chem-net.blogspot.com/2013/06/lewis-dot-structure-thionyl-chloride.html
DeleteWhy the negative charge is on carbon atom?why Will it be wrong if we put it on the nitrogen atom? Please explain.
ReplyDeleteI really enjoyed reading your article. I found this as an informative and interesting post, so i think it is very useful and knowledgeable. I would like to thank you for the effort you have made in writing this article.
ReplyDeletehttps://www.advancedchemistry.in