Simple Procedure for writing the Lewis Structures of CN+
Simple Procedure for writing Lewis Structures of CN+
A simple procedure for writing the Lewis structures of CN+
Let us consider the case of the CN+ cation:
Step 1: Connect the atoms with
single bonds.
Step 2: Calculate the # of electrons in pi bonds (pi bonds, multiple bonds) using formula (1):
Where n in this case is 2 since CN+ consists
of 2 atoms.
Where V = (4 + 5
) - charge = 9 – 1 = 8
Therefore, P = 6n
+ 2 – V = 6 *2 + 2 – 8 = 6 Therefore there are 6 pi electrons (pi electrons). So, there
are 1) either 3 double bonds or 1 triple bond and an unshared electron pair.
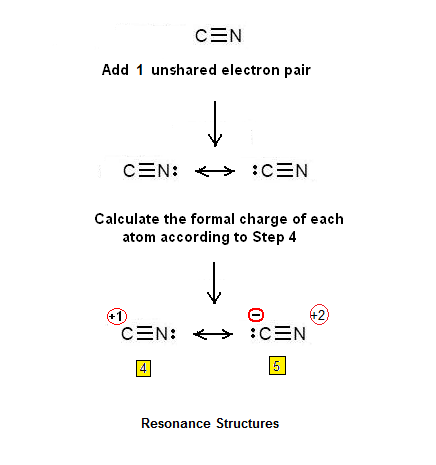
Step 3 & 4: Two atoms cannot be connected with 3 double bonds. However, it is possible that will be connected by a double bond. Two unshared electron pairs will be also added – so that in total 6 pi electrons will be present:
Another possibility is that the two atoms will be connected by a triple bond. In this
case an unshared electron pair will be added so that 6 pi electrons will be
present. The Lewis dot structures of the CN+cation are shown below:
Relevant Posts
Lewis Structures and the Octet Rule - A simple method to write Lewis Structures
Lewis Electron Dot Structures of Hydrogen Cyanide (HCN) and Nitronium Ion (NO2+)
Simple method for writing Lewis Structures – Ozone O3 and carbonate CO3-2
Simple Method for drawing Lewis structures (Video): H2CO3
References
- G.N. Lewis, J.A.C.S, 38, 762-785, (1916)
- E. C. McGoran, J. Chem. Educ., 68, 19-23 (1991)
- A.B.P. Lever, J. Chem. Educ., 49, 819-821, (1972)
Key Terms
electron dot Lewis structures, Lewis Structures and the Octet Rule, how can we draw the CN+ Lewis dot structure

No comments:
Post a Comment