Keto-Enol Tautomerism – Enolization – Reactions and Mechanism
Acid or Base catalyzed Enolization – Reactions and Mechanism
Carbonyl compounds (aldehydes, ketones, carboxylic esters, carboxylic amides) react as electrophiles at the sp2 hybridized carbon atoms and as nucleophiles if they contain an H-atom in the α-position relative to their C=O or C=N bonds. This is because this H is acidic and it can be removed by a base leaving behind an electron pair for nucleophilic attacks.
For most compounds in organic chemistry all the molecules have the same structure – even if this structure cannot satisfactory represented by a Lewis formula – but for many compounds there is a mixture of two or more structurally distinct compounds that are in rapid equilibrium. This phenomenon is called tautomerism.
Tautomerism is the phenomenon that occurs in any reaction that simply involves the intramolecular transfer of a proton. An equilibrium is established between the two tautomers (structurally distinct compounds) and there is a rapid shift back and forth between the distinct compounds.
A very common form of tautomerism is that between a carbonyl compound containing an α-hydrogen and its enol form (Fig. I.1).

Fig. I.1: A keto-enol reaction
An enol is exactly what the name implies: an ene-ol. It has a C=C double bond (diene) and an OH group (alcohol) joined directly to it.
Notice that in the above reaction as in any keto-enol reaction there is no change in pH since a proton is lost from carbon and gained on oxygen. The reaction is known as enolization as it is the conversion of a carbonyl compound into its enol.
Notice also that in the above reaction the product is almost the same as the starting material since the only change is the transfer of one proton and the shift of the double bond.
In simple cases (R2 = H, alkyl, OR, etc.) the equilibrium of the keto-enol reaction lies well to the left (keto structure) (Table I.1). The reason can be seen by examining the bond energies in Table I.2.
Compound |
Enol Content, % |
Acetone |
6 * 10-7 |
PhCOCH3 |
1.1 * 10-6 |
CH3CHO |
6 * 10-5 |
Cyclohexanone |
4 * 10-5 |
Ph2CHCHO |
9.1 |
PhCOCH2COCH3 |
89.2 |
Table I.1: The enol content of some carbonyl compounds
If keto-enol reactions are common for aldehydes and ketones why don’t simple aldehydes and ketones exist as enols?
IR and NMR Spectra of carbonyl compounds show no signs of enols. The equilibrium lies well over towards the keto form (the equilibrium constant k for cyclohexanone is about 10-5).
|
Bond (Energy, kJ/mol) |
Sum ( kJ/mol) |
keto form |
C-H (413) |
C-C (350) |
C=O (740) |
1503 |
enol form |
C=C (620) |
C-O (367) |
O-H (462) |
1449 |
Table I.2: Bond energies in the keto and in the enol form. The keto form is thermodynamically more stable than the enol form by approximately 50 kJ/mol
The approximate sum of the bond energies in the keto form is 1503 kJ/mol while in the enol form 1449. Therefore, the keto form is thermodynamically more stable than the enol form by approximately 50 kJ/mol.
In most cases, enol forms cannot be isolated since they are less stable and are formed in minute quantities. However, there are some exceptions and in certain cases a larger amount of the enol form is present and it can be even the predominant species:
- Molecules in which the enolic double bond is in conjugation with another double bond (cases are shown in Table I.1 like Ph2CHCHO and PhCOCH2COCH3)
- Molecules that contain two or more bulky aryl groups (Fig. I.2). Compound I in Fig. I.2 (a substituted enol) is the major species in equilibrium (~95%) while the keto form is the minor species (~5%). In cases like this steric hindrance destabilizes the keto form (the two substituted aryl groups are 109° apart) while in the enol form 120° apart.
Fig. I.2: A keto-enol reaction. The enol form (I) is the major species in this case since the keto form is destabilized by steric hindrance (the substituted aryl groups are closer in the keto form – the C-C angle is 109° and this is unfavorable due to steric hindrance)
Is there experimental evidence that keto-enol reactions are common for aldehydes and ketones?
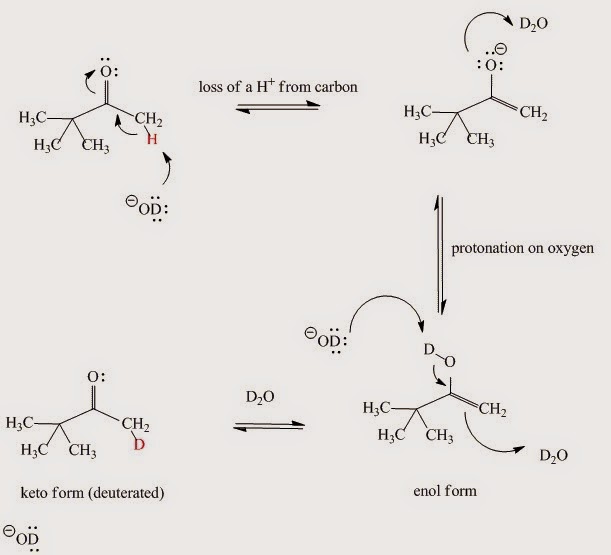
If the NMR spectrum of a simple carbonyl compound in D2O is obtained – such as pinacolone’s (CH3)3CCOCH3 – the signal for protons next to the carbonyl group very slowly disappears. The isolated compound’s mass spectrum (after the above mentioned reaction with D2O is over) shows that those hydrogen atoms have been replaced by deuterium atoms. There is a peak at (M+1)+ or (M+2)+ or (M+3)+ instead of M+. The reaction is shown in Fig. I.3:
Fig. I.3: Evidence for a keto-enol reaction when pinacolone (CH3)3CCOCH3 reacts with D2O. When the enol form of the pinacolone reverts to the keto form it picks up a deuteron instead of a proton because the solution consists almost entirely of D2O.
What mechanism can be proposed for the above reaction?
Enolization is a slow process in neutral solution, even in D2O, and is catalyzed by acid or base in order to happen.
In the acid-catalyzed reaction the molecule is first protonated on oxygen and then loses the C-H proton in a second step (Fig. I.4). When the enol form reverts to the keto – since this is an equilibrium process – it picks up a deuteron instead of a proton since the solution is D2O.
Fig. I.4: The acid-catalyzed keto-enol reaction mechanism. If D2O is the solvent then the α-hydrogens to carbonyl group are replaced by deuterium.
In the base-catalyzed reaction the C-H proton is removed first by the base (for example hydroxide ion OH-, OD- in our case) and the proton (or D+ in our case) added to the oxygen atom in a second step (Fig. I.5).
Fig. I.5: The base-catalyzed keto-enol reaction mechanism. If D2O is the solvent then the α-hydrogens to carbonyl group are replaced by deuterium.
Notice that the enolization reactions in Fig. I.4 and Fig. I.5 are catalytic. In the acid-catalyzed mechanism the D+ (or H+ if water is the solvent) is regenerated at the end (catalyst). In the base-catalyzed mechanism OD- (or OH- if water is the solvent) is regenerated at the end (catalyst).
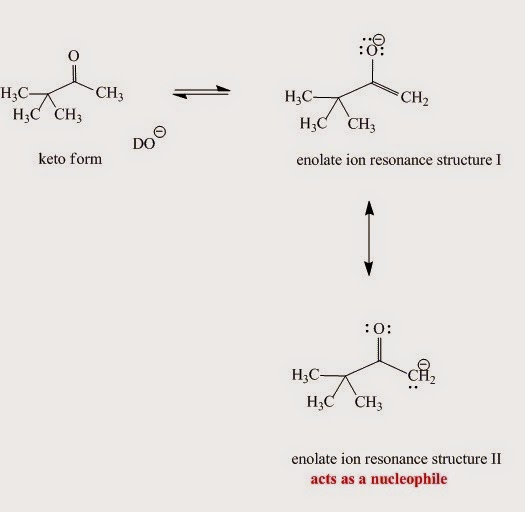
The enolate ion generated from the enol (Fig. I.6) in the base-catalyzed mechanism is nucleophilic due to:
An enolate ion is an ion with a negative charge on oxygen with adjacent C-C double bond.
Fig. I.6: Enolate ion resonance contributors. Although the major contributor is resonace structure I when it reacts as a nucleophile structure II is more reactive.
Enolates are reactive nucleophiles. Although the major enolate Lewis contributor shows concentration of electron density on the electronegative oxygen when it reacts as a nucleophile, it behaves like the electron density is concentrated on the α-carbon next to carbonyl group.
Enolates react with alkyl halides, aldehydes/ketones and esters and these reactions are shown in the post entitled “The chemistry of enolate ions – Enolate ion reactions”.
Relevant Posts
Carbocations: Factors affecting their stability
Carbocations and factors affecting instability
Carbocations: Stability. formation and reactions
References
- A.J. Kresge, Pure Appl. Chem., 63, 213 (1991)
- B. Capon, The Chemistry of Enols, Wiley, NY, 307–322 (1990)
- S.E. Biali et al., J. Am. Chem. Soc. 107, 1007 (1985).
Key Terms
carbonyl compounds, keto-enol reaction, keto form, enol form, α-hydrogen, enolization, enolates, tautomerism