Carbanions are species with a complete octet around the carbon atom. A general formula of a carbanion is of the form:
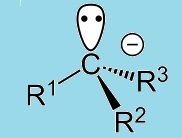
By definition, every carbanion possesses an unshared pair of electrons and is therefore a base. When a carbanion accepts a proton, it is converted to its conjugate acid. The stability of a carbanion is directly related to the strength of its conjugate acid. The weaker the acid, the greater the base strength and the lower the stability of the carbanion - the greater the reactivity of the carbanion. Less stable carbanions tend to react rapidly with proton donors and hence exist as carbanions for short time.
Carbanion stability has been found to be in this order:
vinyl > phenyl > cyclopropyl > ethyl > n-propyl > isobutyl > neopentyl > cyclobutyl > cyclopentyl
References
-
R. Bruckner, “Advanced Organic Chemistry – Reaction Mechanisms”, 2nd Edition, Elsevier, 2002
- M.B. Smith & J. March “March’s Advanced Organic Chemistry”, 6th Edition, Wiley-Interscience, 2007
No comments:
Post a Comment