A simple procedure for writing electron dot structures (ELDOTS) was given in a previous
article entitled “Lewis
Structures and the Octet Rule”.
Several worked examples relevant to this procedure were given in previous articles please see the Sitemap - Table of Contents (Lewis Electron Dot Structures).
Let us consider
the case of dichlorine monoxide Cl2O. What is the
electron dot structure (ELDOTS) for
this molecule?
Dichlorine
monoxide is considered to be an important active chlorinating agent under
typical drinking water and wastewater treatment conditions with hypochlorous
acid HOCl. In a report to the May 2010 issue of Environmental Sci. & Technology
L. Roberts and coworkers of the Johns
Hopkins University report that Cl2O reacts with micropollutants in
source waters, such as the herbicide dimethenamid, to form chlorinated products
that may be more toxic than the parent compounds.
As a matter of
fact M. Reinhard et al. elegantly have demonstrated that Cl2 and Cl2O
played much more important role in the chlorination of organic compounds such
as p-xylene than did bleach HOCl.
Step 1: Connect the atoms with
single bonds. The less electronegative is the oxy gen atom. Hence, the O atom is
going to be the central atom.
Step 2: Calculate the # of electrons in π bonds (multiple bonds) using Lewis structure formula (1):
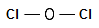 |
|
Fig.
1 : Connect the atoms of Cl2O with single bonds.
|
Step 2: Calculate the # of electrons in π bonds (multiple bonds) using Lewis structure formula (1):
Where n in this
case is 3. Where V = (7 +6 + 7 ) = 20 ,
V is the number of valence electrons of the Cl2O molecule.
Therefore, P = 6n
+ 2 – V = 6 * 3 + 2 – 20 = 0 \ So, there is no double bond.
Step 3 & 4: The electron
dot structures (ELDOTS)
of Cl2O are as follows:
 |
| Fig. 2 : Electron dot structures (ELDOTS) of Cl2O. |
Thanks! This was helpful!
ReplyDelete