gradient elution in liquid chromatography LC
Gradient Elution in Liquid Chromatography
Gradient elution is used in chromatography (mainly in reversed-phase but with other modes such as ion-exchange) to modify the separations achieved in a column. It involves a continuous change in the composition of the mobile phase to achieve separation of sample components of widely varying affinities for the stationary phase.
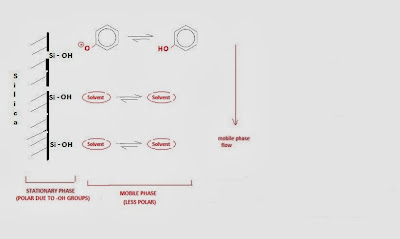
The separation in a chromatographic column can be changed by changing the polarity of either the column (stationary phase) or the mobile phase. Generally, it is easier and cheaper to change the composition of the mobile phase (solvent).
The method used more often to modify the separation is to change the difference in polarity between the stationary phase and the mobile phase.
The following is observed for the polarity difference of these phases:
- Increasing the difference in polarities between the stationary and the mobile phase makes compounds elute slower – they adsorbed more efficiently on the column.
- Making the solvent polarity more like the column polarity makes compounds elute faster
For example:
On a nonpolar column running in acetonitrile if we could switch to a more polar mobile phase (by adding a known percentage of water –which is very polar- to the acetonitrile solution) then the compounds will be retained longer in the stationary phase and they will have more time to separate.
We could also start with a mobile phase containing a large percentage of water to make nonpolar compounds stick to the top of the column and then gradually increase the amount of acetonitrile to elute them (Fig. I.1).

Relevant Posts
What is reversed phase liquid chromatography (LC) / HPLC?
Normal phase liquid chromatography (LC) / HPLC?
Which are the main liquid chromatography separation modes? / Liquid-Liquid Chromatography (LLC)
Selection of the Liquid Chromatographic (LC) / HPLC Separation Mode
Troubleshooting HPLC / Liquid Chromatography Systems – Peak Tailing
References
- Nina Hadden et al., “Basic Liquid Chromatography”, Varian Aerograph, 1971
- C.A. Dorschel, J.L. Ekmanis, J.E. Oberholtzer et al. “LC Detectors” Anal. Chem., 61, 951A–968A, 1989
- C. F. Simpson, “Techniques in Liquid Chromatography” Wiley-Hayden: Chichester, England, 1982
- L.R. Snyder, J. L Glajch, J. J. Kirkland, “Practical HPLC Method Development”, Wiley-Interscience: New York, 1988.
- V.R. Meyer, “Practical High – Performance Liquid Chromatography”, Wiley, 2010
- M. McMaster, “LC/MS A Practical User’s Guide”, Wiley-Interscience, 2005
Key Terms
gradient elution, chromatography, mobile phase, polarity difference, polar mobile phase, separation in chromatographic column, capillary column,