Food Chemistry: Sulfites and SO2 as food preservatives
Preservatives or antimicrobial agents play an important role in today's supply of safe and stable foods. Increasing demand for long self-life of processed foods makes the use of chemical food preservatives necessary. The activity of these preserving agents covers yeasts, molds and bacteria. The activity increases with decreasing pH and is mostly derived from undissociated sulfurous acid, which predominates at a pH < 3.
The use of sulfites as food preservatives in wine for example dates back to ancient times. In our days, sulfur dioxide - a gas sold in cylinders - can be injected directly in liquids or it can be dissolved in ice-water to form sulfurous acid. Most of the time though, instead of sulfur dioxide solutions, a number of sulfites are used (Table I.1) because, when dissolved in water, they all yield active SO2.
Name |
Formula |
% Active SO2 |
Sulfur dioxide |
SO2 |
100 |
Sodium sulphite, anh. |
Na2SO3 |
50.80 |
Sodium hydrogen sulfite |
NaHSO3 |
61.56 |
Sodium metabisulfite |
Na2S2O5 |
67.39 |
Potassium metabisulfite |
K2S2O5 |
57.63 |
Table I.1: Common sulfites that are used as food preservatives
The most widely used of the above sulfites is potassium metabisulfite. Sulfite (bisulfite ion HSO3-) reacts with a series of food constituents e.g. proteins with cleavage of disulfide bonds, folic acid, dextrins, aldehydes and ketones to form addition compounds (Fig. I.1):
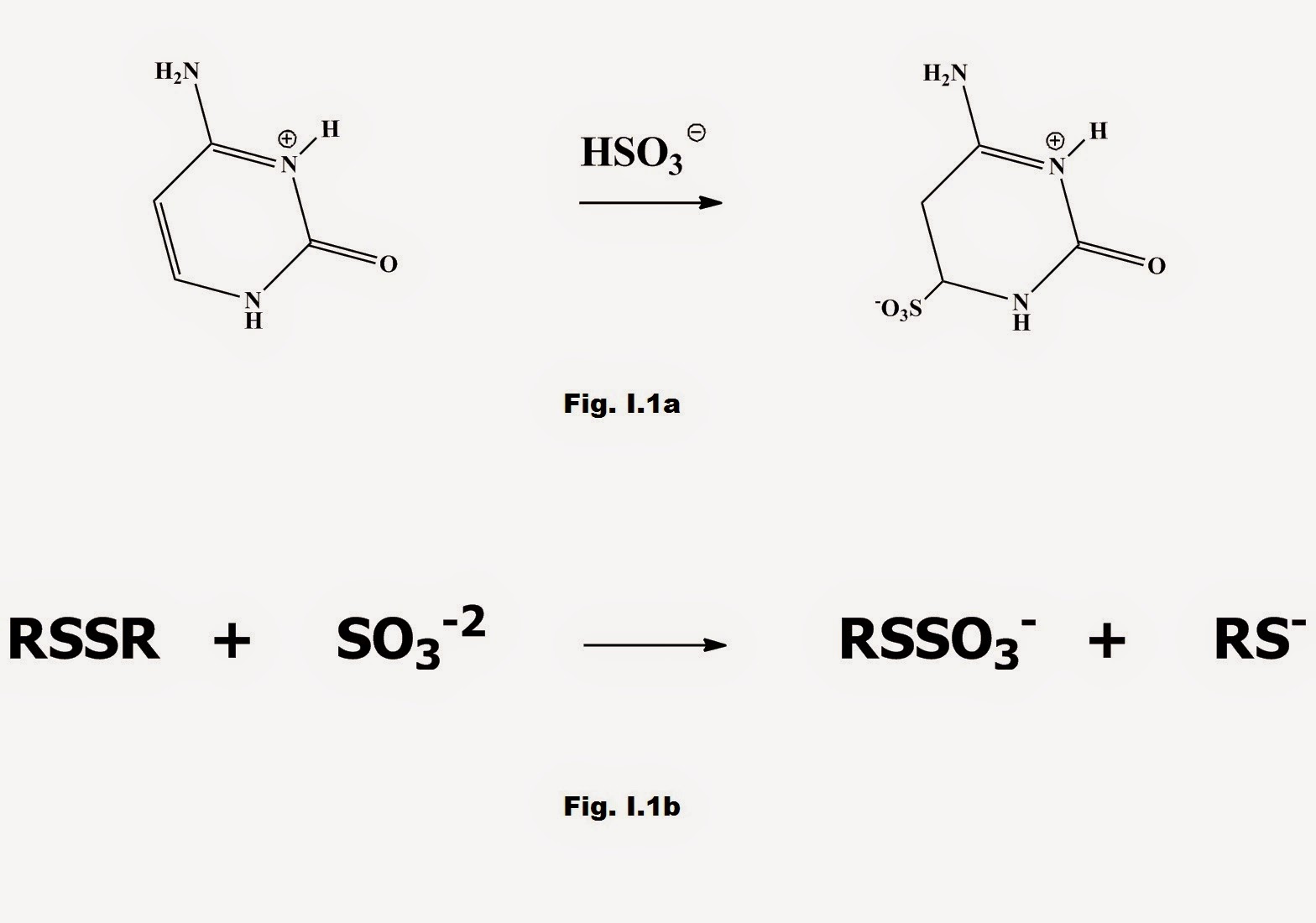
Fig. I.1 a) Pyrimidines in nucleic acids react with HSO3- to form addition compounds b) Cleavage of S-S bonds in aminoacids by SO3-2 to form addition compounds
The addition compounds of sulfites are known as bound sulfur dioxide. Sulfur dioxide is used extensively in wine making, and in wine acetaldehyde reacts preferentially with bisulfite. Excess bisulfite reacts with sugars. It is possible to classify bound SO2 into three forms:
• aldehyde sulfurous acid
• glucose sulfurous acid
• rest sulfurous acid (holds the SO2 in a less tightly bound form)
Crushed grapes or must are treated with sulfur (by the addition of sulphites or an aqueous solution of sulfurous acid or by adding liquid SO2) immediately after grape crushing to preserve the constituents that are sensitive to oxidation, prevent enzymatic browning via phenol oxidation and suppress the growth of undesirable microorganisms (acetic acid bacteria, molds). Another important effect is the suppression of undesirable aroma notes by the binding of carbonyl compounds, especially of ethanal, as hydroxysulfonic acids. Therefore, sulfites in wines serve a dual purpose: antiseptic or bacteriostatic and antioxidant.
The maximum quantities of SO2 added to foods is self-limiting because at levels from 200 ppm the product may develop an unpleasant off-flavor. The acceptable daily intake is set to 1.5 mg/kg body weight. Although some other compounds – such as sorbic acid and ascorbic acid – have been used as alternatives to partially replace SO2 the results were not satisfactory.
SO2 is also widely used in dried fruits, where levels may be up to 2000 ppm. Other applications are in dried potato products and in dried vegetables. Because SO2 is volatile and easily lost to the air, the residual levels may be much lower than the amounts originally applied.
Sulfite and SO2 are not only antimicrobially active, but inhibit discoloration by blocking compounds with a reactive carbonyl group (Maillard reaction - nonenzymic browning) or by inhibiting oxidation of phenols by phenol oxidase enzymes (enzymatic browning).
Relevant Posts
Food Chemistry: Chemical reactions in cooking
Food Chemistry: Antioxidants and Oxidation Reactions in Foods
References
- H-D. Belitz et al., “Food Chemistry”, 4th Edition, Springer Verlag, 2009
- P. Barham et al., Chem. Rev., 110, 2313 (2010)
- P. Walstra, "Physical Chemistry of Foods", Marcel Dekker Inc., 2003
- J.M DeMan, "Principles of Food Chemistry", 3rd Edition, Aspen Publishers Inc., 1999
Key Terms
preservatives, food chemistry, molecular gastronomy, Maillard reaction, antimicrobial agents, chemical food preservatives, preserving agents, sulfites as preservatives, active SO2
No comments:
Post a Comment